
Many meat and diary products contain low levels of antibiotics. Image: Shutterstock
Antibiotics may promote fat growth and alter the human microbiome.
Human antibiotics have been widely used as growth promoters to fatten up farm animals since the late 1940s, breeding them to almost double their normal size and much faster. The mechanism as to how antibiotics make this possible remains unclear, as do the health effects associated with the consumption and handling of antibiotic-fed livestock.
Studies have started to paint a picture of how exposure to low doses of antibiotics can cause diverse health problems including allergies, bacterial resistance, gastrointestinal problems and more recently, obesity. In a paper published in Nature, scientists at New York University have shown that mice exposed to the same amount of antibiotics received by farm animals develop 15 per cent more body fat. Dramatic changes in gut microbial communities and genetic profiles of the mice were also observed.
In another study published in the International Journal of Obesity (IJO), a different group of researchers investigated the effects of antibiotics exposure in a cohort of over 10,000 human babies under six months old. They found that the babies treated with antibiotics were 22 per cent more likely to become overweight in childhood and later life. Although there is a clear public health importance in determining the link between adiposity, microbiome disruption and antibiotics, the field has previously remained curiously unexplored. However, these two recent studies raise the alarm on a cause of obesity other than the usual culprits of unhealthy diet, sedentary lifestyle and genetics.
“There are several diseases associated with antibiotic exposure, such as Clostridium difficile infections. The rise of antibiotic resistant bacteria is also a significant issue that modern medicine faces,” explains Ilseung Cho, lead author of the study published in Nature. “Our research demonstrates that the microbiome plays a significant role in host health and disease. Though this study focused on adiposity, I believe that the microbiome may have far-reaching effects including [those] on cardiovascular health, colorectal cancers, and inflammatory bowel diseases.”
Babies who are given antibiotics at less than six months old are the most vulnerable because their immune system and microbiome has not yet fully developed. “Early life appears to be a critical period for gut colonisation, with interruption of normal colonisation (for example, bottle feeding or early administration of antibiotics) disrupting ancient patterns of intestinal colonisation,” say the authors of the study published in IJO.
Feeding animals antibiotics has economic benefits for farmers, meat companies and also consumers. But with more studies being conducted on the topic it is becoming clear that the long-term public health costs and ethical problems associated with speeding up livestock growth with antibiotics probably outweigh the financial profits. That said, Cho explains that antibiotics are still important when used judiciously and people should continue to take them when prescribed by their doctor.
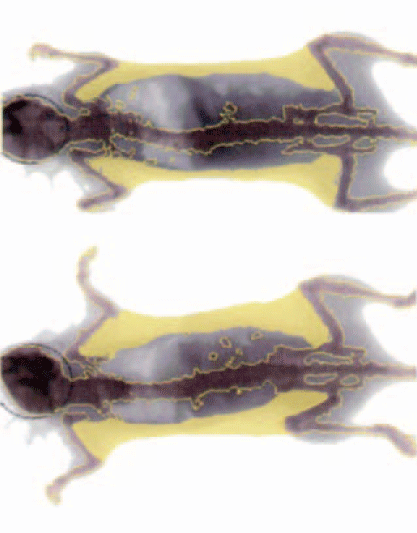
Comparison of body fat (yellow) between mice unexposed (top) and exposed (bottom) to continual low doses of antibiotics. Image: Cho et al., Nature 2012
Source: Eurekalert






