More than 180 different COVID-19 vaccines were in the initial pipeline – and the majority of them can be placed in one of four vaccine categories. One is a classic. Two are comparatively recent. And one has never before been approved for use in humans.
Normally, it takes 10-15 years to develop a new vaccine. The all-time record was four years. For SARS-CoV-2, it was achieved in only one year – and with more than 180 competitors in action.
Scientists not only had to work extremely fast, they also to choose the most efficient method for producing their possible COVID-19 vaccine to make the immune system produce antibodies and T cells that could combat the virus.
The classic method for a vaccine is to use an inactivated or weakened version of the virus.
Weakened virus
SARS-CoV-2’s genetic material (white) is destroyed by high temperatures. The virus exterior remains intact and so trains the immune system to recognise the real virus. The method is familiar, but in rare cases the weakened virus could cause disease.
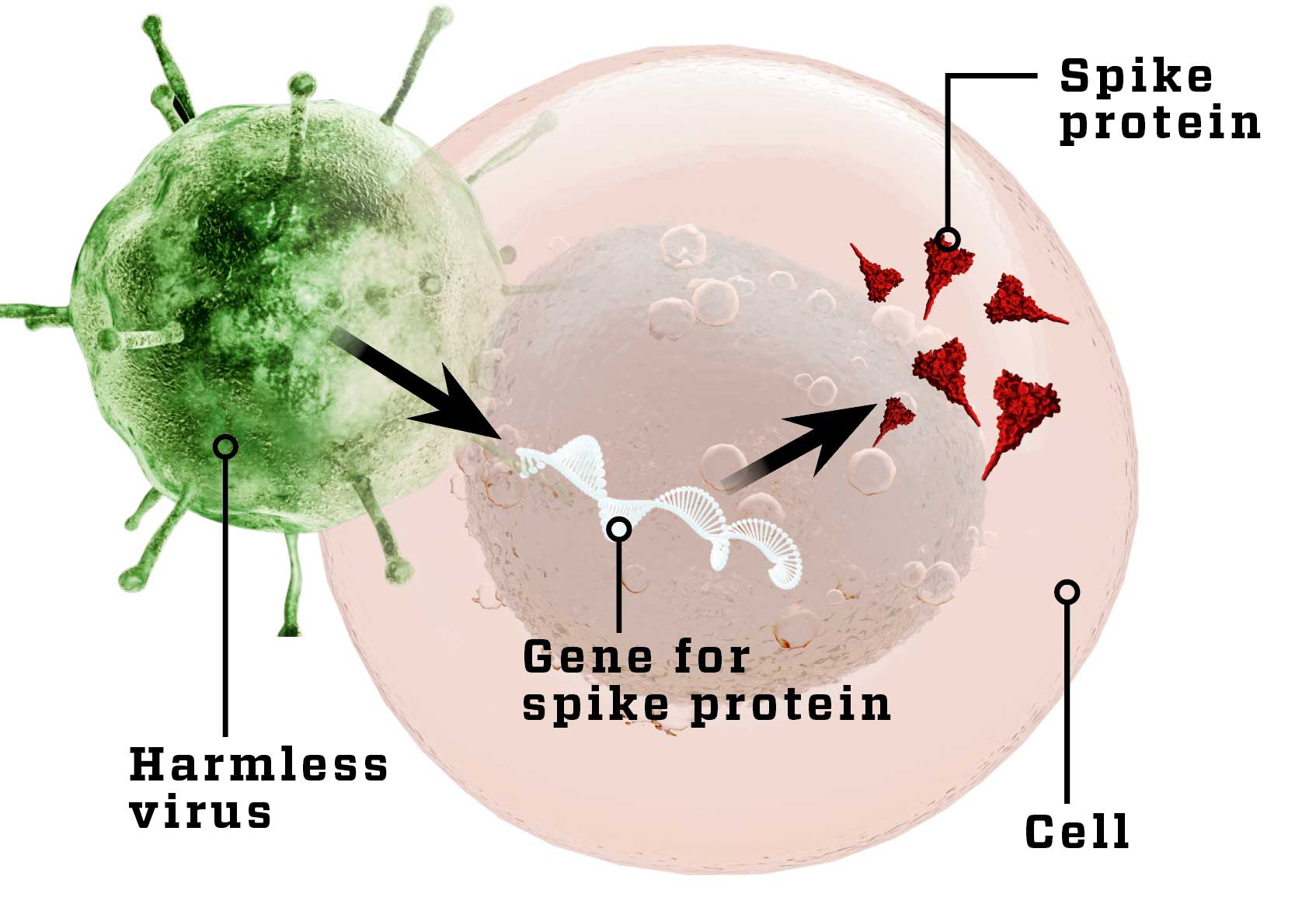
Harmless virus
A harmless virus (white) with the gene for SARS-CoV-2’s spike protein makes body cells produce the spike protein (dark triangles), so the immune system gets to know it. In some cases, the body begins to fight the harmless virus.
But with these types of vaccine, scientists cannot control at which parts of the virus the immune system will aim its antibodies.
Ideally, the antibodies must be aimed at a protein on the surface of the virus that exists only on this specific virus, so that the immune system doesn’t waste energy attacking other harmless viruses. Scientists had previously solved that problem by making a vaccine consisting of that specific protein. That method certainly works, but it is complex and time-consuming to develop.
Virus fragments are expensive to run
Scientists take a protein from the surface of SARS-CoV-2 and inject it directly into the body, so the immune system is trained to recognise the virus. However, such proteins can be difficult and expensive to make in sufficient quantities.
German drug maker BioNTech decided on a new approach. The company already specialised in immune therapy against cancer – a type of treatment that is similar to vaccines. In both cases, the immune system is stimulated to combat the enemy. One of their preferred methods was the use of mRNA molecules. These are similar to DNA, and like DNA they can carry a genetic code that cells can use as a blueprint to produce a protein. By injecting mRNA with the code for a specific virus protein, the scientists could make the body’s own cells produce the protein and hence train the immune system to recognise the virus.

RNA vaccine fast but untested
The gene (white) for the spike protein is injected into the body in the shape of mRNA or DNA, causing the body to produce the protein (dark triangles), so the immune system gets to know it. The method is easy and efficient, but brand new.
BioNTech’s expertise with the mRNA method made Sahin believe that a COVID-19 vaccine could be developed the same way. But other scientists had previously experimented with mRNA vaccines, and nobody had yet succeeded. However, the potential advantages were obvious. An mRNA vaccine can be designed and made in the lab in less than a week, whereas it takes months to make more conventional vaccines. So by early February BioNTech had already made a series of prototypes of the vaccine that they could test on animals. Rhesus macaques and mice had a number of mRNA molecules injected into their bodies, and the scientists studied how the animals’ immune systems reacted, and whether the animals became immune to coronavirus.
By early April, these animal trials had been completed, and a vaccine candidate with the code name of BNT162b2 looked like a winner. The vaccine’s mRNA molecule made the immune system attack part of the spike protein on the surface of coronavirus, which the virus uses to enter body cells. One single injection of BNT162b2 made the treated animals produce sufficient quantities of both antibodies and T cells to attack SARS-CoV-2. And the vaccine protected the animals efficiently against pneumonia.
With these promising results, BioNTech teamed up with big American drug company Pfizer, and in late April, they were allowed to begin human trials in both Germany and the United States.
In the US, the companies Moderna and Inovio were already busy testing vaccines on humans. Like BioNTech, Moderna had used mRNA in its vaccine, whereas Inovio used DNA. In China, the company CanSino Biologics was testing another vaccine, this one consisting of a harmless cold virus that made body cells produce coronavirus spike protein.
Swedish-British firm AstraZeneca had also developed a vaccine – following the same methodology as CanSino Biologics’ – and on 22 May 2020, its vaccine was the first to reach final phase 3 trials on humans. At that time, there were 19 different vaccines being tested in experiments on humans across the world, while 130 other vaccine candidates were being tested on animals. Only AstraZeneca had reached phase 3, in which the vaccine is tested on thousands of people.
In cooperation with the UK’s University of Oxford, the company chose to carry out its trials in Brazil, where the number of new COVID-19 cases was growing day by day. Because the 40,000 test subjects were facing to a high infection rate, the scientists had an excellent opportunities to test if the vaccine was efficient.
By early November, BioNTech and Pfizer took over the lead in the vaccine race, the first to publish the preliminary results of their crucial phase 3 experiments. At that time, a total of 94 of the 43,000 test subjects had tested positive for coronavirus – 86 of them from the placebo group which had not received the vaccine, and only eight from the vaccinated group. The scientists were thereby able to conclude that the vaccine offered higher than 90% protection against infection with SARS-CoV-2. The vaccine was a success. As more test results were received the result was adjusted to 95% protection.
Only one week later, Moderna reported equally good results for its mRNA vaccine candidat. And in late November, AstraZeneca finished its vaccine trials. It scored only 70% for protection, but on the other hand it can be stored at fridge temperatures instead of the -70°C freezing required by its competitors.
15 years of work done in one year
Normally it would take 10-15 years to develop a new vaccine and bring it to market. But with efficient cooperation between drug makers and health authorities, the COVID-19 vaccine was developed in less than a year – without compromising safety.

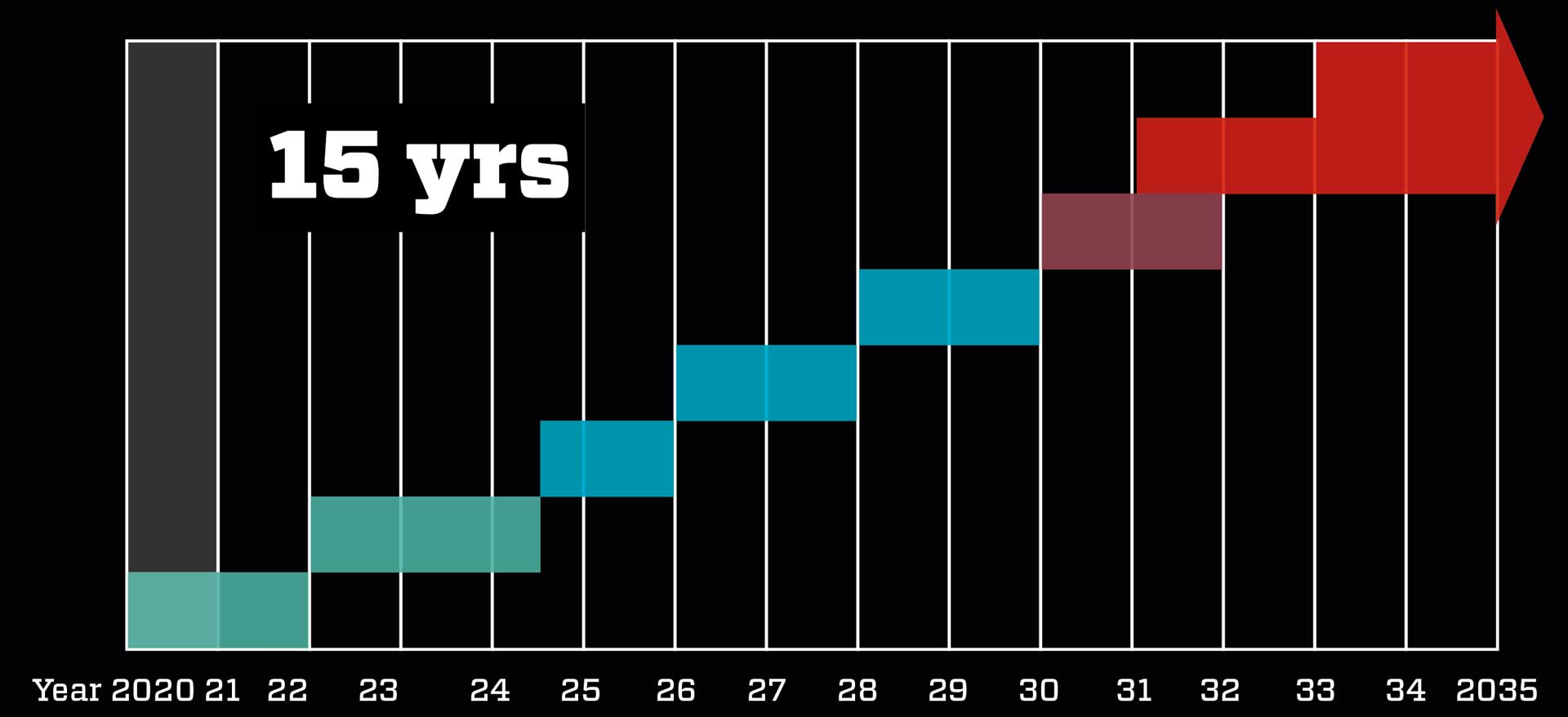
Normal procedure takes about 15 years: Scientists develop the vaccine in the lab, test it on animals, and then test in on humans in three phases – on first 50, then 300, and finally 30,000 test subjects. The trials are very expensive, so under normal circumstances the company behind the vaccine takes breaks between the phases to calculate whether it is worthwhile continuing. The final result is then introduced to the authorities, who decide whether the vaccine is to be approved. Factories can then be equipped to make the vaccine, followed by global distribution.
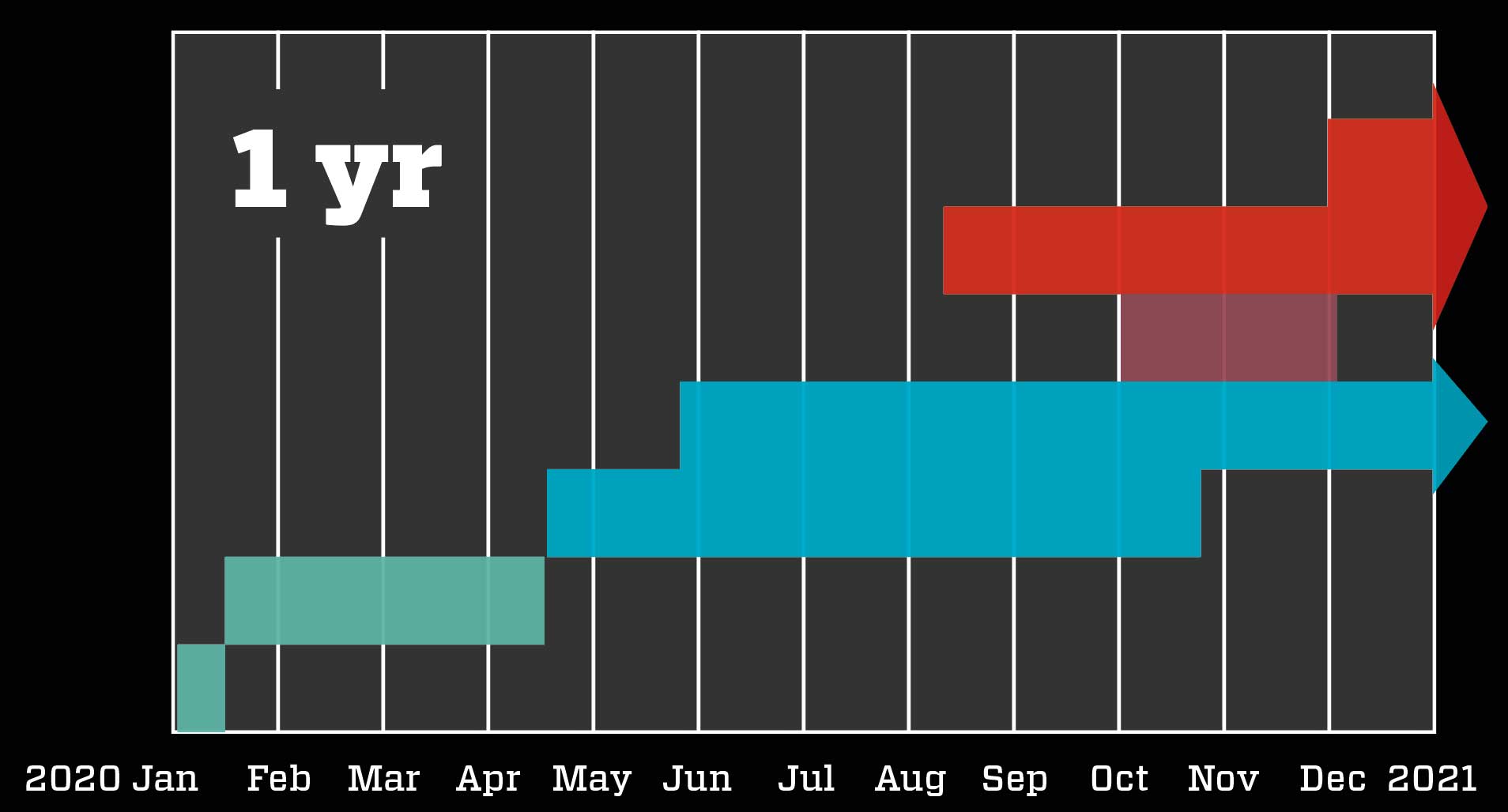
Fast procedures speed up the process: Scientists quickly develop the vaccine based on knowledge of the new coronavirus, SARS and MERS. The trial phases on animals and humans are completed rapidly and with overlaps because – due to the severity of the situation – authorities contribute millions to the development, so drug makers need not fear major losses. The authorities evaluate the preliminary results and buy millions of doses so mass production can begin. The vaccine is approved before the trials are fully completed, and quickly distributed.
The authorities followed the race as it happens
The health authorities didn’t stand idly by during the vaccine race. Whereas normally, they would not begin evaluating a vaccine until after the final phase 3 trial results were available, the severity of the situation dictated that they followed a much more rapid strategy – though doing so without compromising safety.
Known as a rolling review, the faster procedure involves drug makers continously submitting all preliminary results to the health authorities, which can thereby get an ongoing general idea of the vaccine’s efficiency, any side effects, and similar critical results – all long before the phase 3 trials are fully completed. This allows the authorities to make a qualified decision about approval only a few weeks after receiving the final results. And that is exactly what happened to BioNTech and Pfizer with their vaccine.
The companies submitted final research results on 18 November, and on 2 December the vaccine was already approved in the UK. On 11 December, it was approved in the US, and on 21 December in the EU. Australia gave it provisional approval on 25 January 2021. At the same time, preparations for vaccination were under way. Doses had already been manufactured, the authorities had bought and paid for them, and the infrastructure behind the distribution of the vaccines was being put into place. The EU’s first injections occurred only six days after their approval htere; the first Australian vaccination took place on the morning of February 21st.
Competitors cooperated
In the wake of Pfizer’s and BioNTech’s vaccine, the vaccines from Moderna and AstraZeneca were also approved. Many more vaccines are in the pipeline – which is a good thing. No single company could manufacture enough doses of vaccine to innoculate the whole world within a reasonable time frame. Also, the vaccines based on different principles may deliver separate strengths and weaknesses. Scientists expect that some vaccines will be more efficient for the elderly, others for young people. Some vaccines might offer very high protection that lasts for a relatively short period of time, whereas others may offer a lower level of protection but over much longer periods. Scientists do not yet know which vaccines will perform best in different scenarios.
As scientists gradually answer such questions, different health authorities will be able to develop the best strategy for their struggle against coronavirus. Scientists still have much work to do, even in connection with the approved vaccines. Now, they need to monitor who gets which vaccines when, and how many still get ill. Further research is needed on the emerging variants of SARS-CoV-2, and on how long the benefits of vaccination will last.