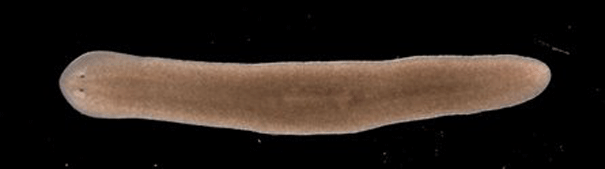
This simple worm could theoretically live forever. Image: University of Nottingham
Forget botox injections and age-defying creams, a species of flatworm may hold the key to looking young forever.
Researchers at the University of Nottingham have demonstrated how planarian worms have an apparent limitless ability to regenerate by replacing aged or damaged tissues and cells. In normal cases, when stem cells divide they begin to show signs of ageing and are no longer able to divide effectively.
As a result, they become less capable of replacing specialised cells in the tissues of our bodies, which is why human skin visibly ages. The stem cells of planarian worms, however, are able to avoid this ageing process and keep their cells dividing.
“We’ve been studying two types of planarian worms; those that reproduce sexually, like us, and those that reproduce asexually, simply dividing in two,” Dr Aziz Aboobaker said. “Both appear to regenerate indefinitely by growing new muscles, skin, guts and even entire brains over and over again.”
When a cell divides and copies DNA, the strands of DNA get snipped to enable the copying process. The places that are snipped are called telomeres, which continue to get shorter each time a cell divides.
When they get too short, the cell loses its ability to renew and divide. In humans, a cell replicates around 50 times before the telomere becomes too short, but in an “˜immortal’ animal, we would expect the cell replication to be limitless.
Previous work had shown that an enzyme called telomerase could maintain telomeres. However in most sexually reproducing organisms the enzyme is most active only during early development, so the telomeres start to become shorter as we age.
The researchers identified a possible planarian version of this enzyme, and were able to turn down its activity. The results showed a reduction in telomere length, proving it was the right enzyme.
“Our data satisfy one of the predictions about what it would take for an animal to be potentially immortal and that it is possible for this scenario to evolve,” Aboobaker said.
This discovery, published in the Proceedings of the National Academy of Sciences, could shed light on alleviating ageing and age-related characteristics in human cells. “This exciting research contributes significantly to our fundamental understanding of some of the processes involved in aging, and builds strong foundations for improving health and potentially longevity in other organisms, including humans,” Professor Douglas Kell, Chief Executive at Biotechnology and Biological Sciences Research Council, said.
-Alice Orszulok
Source: University of Nottingham